|
Upregulated expression of interleukin-8, RANTES and chemokine receptors in human astrocytic cells infected with HIV-1
Manuela Cota1,
Andrea Kleinschmidt4,
Francesca Ceccherini-Silberstein4,
Francesca Aloisi5,
Manuela Mengozzi1,
Alberto Mantovani2,3,
Ruth Brack-Werner4,6 & Guido Poli1,6
1AIDS Immunopathogenesis Unit, DIBIT, San Raffaele Scientific Institute, 20132 Milano, Italy
2Department of Immunology and Cell Biology, `Mario Negri' Institute for Pharmacological Research, 20157 Milano, Italy
3Section of Pathology and Immunology, Department of Biotechnology, University of Brescia, 25123 Brescia, Italy
4Institute of Molecular Virology, GSF-National Research Center for Environment and Health, 85764 Neuherberg, Germany
5Laboratory of Organ and System Pathophysiology, Istituto Superiore di Sanità, 00161 Roma, Italy
Correspondence to: M Cota, P2-P3 Laboratories, DIBIT, Via Olgettina n. 58, 20132 Milano, Italy
6R Brack-Werner and G Poli contributed equally to this study
|
Human immunodeficiency virus (HIV) infection of the central nervous system (CNS) affects primarily microglial cells and astrocytes. Infection of these latter cells occurs independently of CD4 and is characterised by preferential accumulation of 2 Kb mRNA, encoding mostly Nef, and by low levels of 4.5 and 9 Kb RNAs. We have investigated the potential role of chronic HIV infection of human astrocytic cells on the expression of pro-inflammatory cytokines, chemokines and their receptors by comparing the infected TH4-7-5 with its parental uninfected 85HG66 cell lines. Upregulated levels of tumour necrosis factor- (TNF- (TNF- ) and of certain chemokines, namely interleukin-8 (IL-8) and regulated upon activation normal T cell expressed and secreted (RANTES), were observed in the infected versus uninfected cells, whereas monocyte chemotactic protein-1 (MCP-1) was comparably expressed in both cell lines. This pattern of expression was confirmed in primary foetal astrocytes transiently transfected with HIV. In addition, CXCR1, CXCR2 and CCR2b, receptors for IL-8 and MCP-1, respectively, were also found to be upregulated in TH4-7-5 versus 85HG66. CXCR4, the receptor of stromal cell derived factor-1 (SDF-1) and co-receptor for syncytium inducing HIVs, was comparably expressed in infected and uninfected astrocytic cells, whereas CCR5 was not detected in either cell line. Furthermore, treatment of TH4-7-5 cells with TNF- ) and of certain chemokines, namely interleukin-8 (IL-8) and regulated upon activation normal T cell expressed and secreted (RANTES), were observed in the infected versus uninfected cells, whereas monocyte chemotactic protein-1 (MCP-1) was comparably expressed in both cell lines. This pattern of expression was confirmed in primary foetal astrocytes transiently transfected with HIV. In addition, CXCR1, CXCR2 and CCR2b, receptors for IL-8 and MCP-1, respectively, were also found to be upregulated in TH4-7-5 versus 85HG66. CXCR4, the receptor of stromal cell derived factor-1 (SDF-1) and co-receptor for syncytium inducing HIVs, was comparably expressed in infected and uninfected astrocytic cells, whereas CCR5 was not detected in either cell line. Furthermore, treatment of TH4-7-5 cells with TNF- or IL-1 or IL-1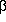 stimulated RNA and protein secretion of IL-8, MCP-1, and RANTES as well as HIV expression. Thus, our findings suggest that HIV infection of astrocytic cells can contribute to the establishment of a chronic inflammatory state in the CNS, eventually resulting in HIV encephalitis, by increasing the secretion of pro-inflammatory cytokines, such as TNF- stimulated RNA and protein secretion of IL-8, MCP-1, and RANTES as well as HIV expression. Thus, our findings suggest that HIV infection of astrocytic cells can contribute to the establishment of a chronic inflammatory state in the CNS, eventually resulting in HIV encephalitis, by increasing the secretion of pro-inflammatory cytokines, such as TNF- and several chemokines. Overexpression of chemokine receptors including CCR2b, CXCR1 and CXCR2 in infected astrocytic cells may contribute to HIV-induced damage of the CNS via autocrine/paracrine activation of astrocytes. Journal of NeuroVirology (2000) 6, 75 - 83. and several chemokines. Overexpression of chemokine receptors including CCR2b, CXCR1 and CXCR2 in infected astrocytic cells may contribute to HIV-induced damage of the CNS via autocrine/paracrine activation of astrocytes. Journal of NeuroVirology (2000) 6, 75 - 83. |

